Sink Conditions Dissolution
Non-sink conditions represented a very discriminating dissolution conditions acting as a sort of magnifier lens for an in-depth evaluation of the dissolution phenomenology and dissolution tests under non-sink conditions can be a pr edictive tool during formulation development as well as for. Sink condition is the ability of the dissolution media to dissolve at least 3 times the amount of drug that is in your dosage form.
For compounds with poor aqueous solubi-lity maintaining sink conditions can be problematic render-ing complete dissolution characterization a challenging task.

Sink conditions dissolution. Dissolution testing should be carried out under mild test conditions basket method at 50100 rpm or paddle method at 5075 rpm at 15-minute intervals to generate a dissolution profile. Sink Conditions If you put a spoon of sugar into a beaker of water it will dissolve readily. Sink conditions affect the production of the sample but not the condition of the solution upon sampling.
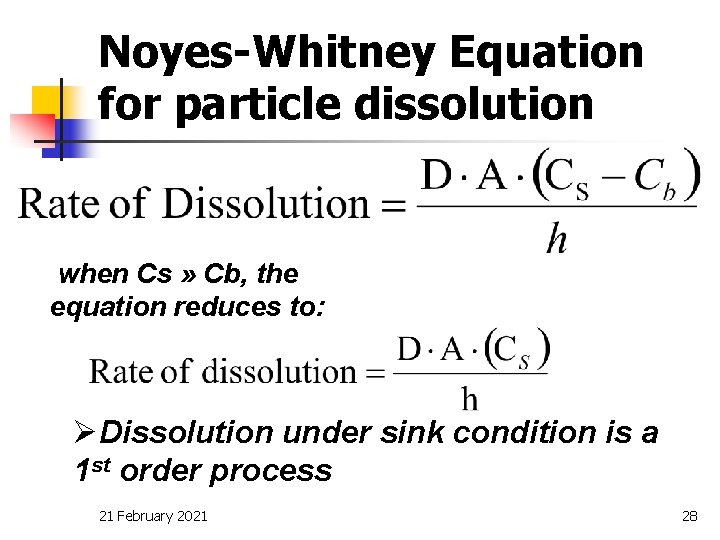
Sink conditions affect the production of the sample. Sink conditions re fer to the excess solubilizingca p a c i ty of the dissolution mediumMost source sre commend at least 3X three times the vo l u m eneeded to co m p l e tely solubilize the dose and some o u rces re commend 5X and even 10XBut howmuch is really needed. A theoretical treatment of zero and first-order dissolution under perfect sink and nonsink conditions is presented.
Sink conditions see Ph. The selection of a suitable dissolution medium composition volume should be based on the physico-chemical characteristics of the active substances and the intended dose range of the drug product and the formulation to be tested. But keep adding spoonfuls and it becomes slower for the sugar to dissolve until at some point it becomes impossible for any more to dissolve as the solution becomes saturated.
To afford sink conditions several solubility modiers such as surfactants inorganic salts and organic co. Conventional dissolution testing remains critical in drug development. The standard dissolution testing conditions described herein.
Having sink conditions helps your dissolution have more. The importance of approximating perfect sink conditions for the determination of dissolution rates which may be correlated with in vivo results is pointed out. A second spoon will also dissolve.
Sink condition A Sink conditions describe a dissolution system that is sufficiently dilute so that the dissolution process is not impeded by approach to saturation of the compound of interest. Generally when developing a dissolution procedure one goal is to have sink conditions defined as the volume of medium at least three times that required in order to form a saturated solution of drug substance. Sink condition A Sink conditions describe a dissolution system that is sufficiently dilute so that the dissolution process is not impeded by approach to saturation of the compound of interest.
Excipients Excipients chosen for drug product formulation should be consistent with the design of IR drug. When sink conditions are present it is more likely that dissolution results will reflect the properties of the dosage form. With recent advances in the development of supersaturating oral dosage forms for poorly water-soluble drugs pharmaceutical scientists are increasingly applying in vitro dissolution testing under non-sink conditions for a direct evaluation of their ability to generate and maintain supersaturation as a predictive surrogate for ensuring product quality and in vivo performance.
Sink Conditions- maintaining a volume of dissolution media that is 5 to 10 times greater than the volume at the saturation point of the drug contained in the drug delivery system being tested as defined in the DDG book volume 1 Not maintaining sink conditions is a bad thing. Sink where V is the dissolution medium volume Cis the saturated solubility of the compound in the medium and Sink is sink condition calculated as CSCD where Cis the concentration of compound in the bulk medium and should be greater than or equal to 3.

Dissolution Testing How Does It Work Youtube

Fig So We Have To Maintain Sink Condition In In Vitro This Is Can Be Download Scientific Diagram
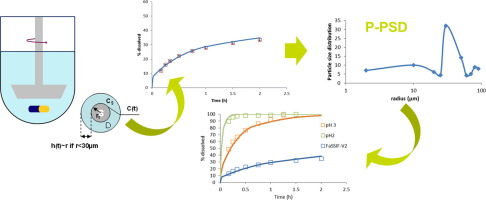
Bridging In Vitro Dissolution And In Vivo Exposure For Acalabrutinib Part I Mechanistic Modelling Of Drug Product Dissolution To Derive A P Psd For Pbpk Model Input European Journal Of Pharmaceutics And Biopharmaceutics

Importance Application Factors Affecting Dissolution Rate Theories Of Dissolution Official Dissolution Tests Ppt Download
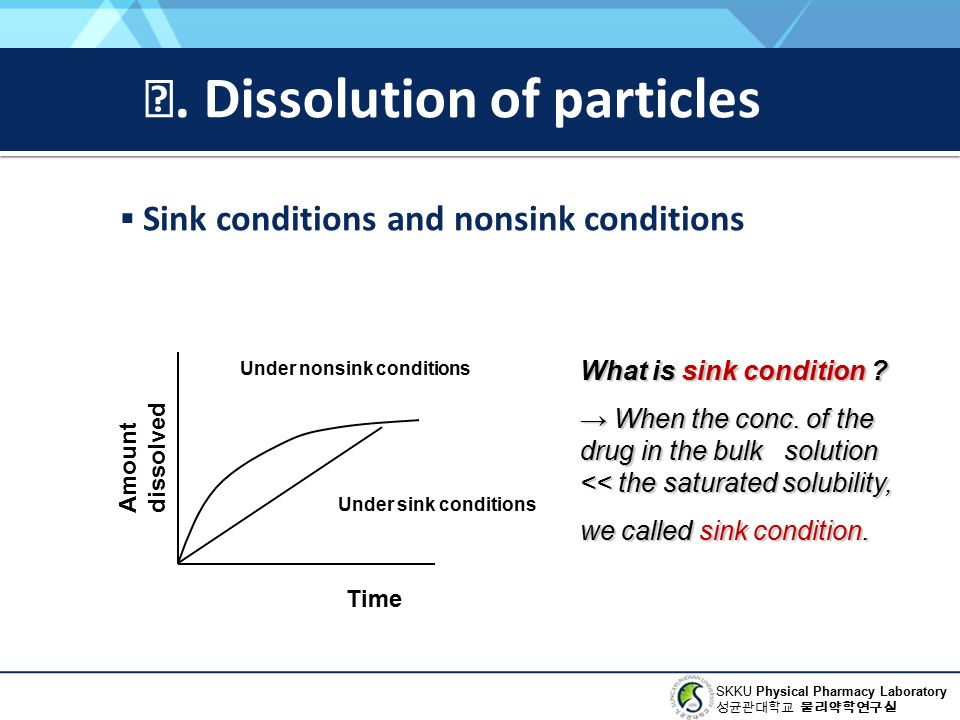
Chapter 13 Dissolution Phenomena Ppt Video Online Download

Dissolution Study Dissolution Studies Factor Affecting Dissolution An
What Is Sink Condition In Dissolution

Pharmaceutics Calculations Flashcards Quizlet

Sink Condition Non Sink Condition Youtube

12 03 Dissolution Flashcards Quizlet

Non Sink Dissolution Conditions For Predicting Product Quality And In Vivo Performance Of Supersaturating Drug Delivery Systems Journal Of Pharmaceutical Sciences
Shear Rate Sink Conditions In Dissolution Testing







Posting Komentar untuk "Sink Conditions Dissolution"